Chemical bonds: definition, types, and examples Igcse chemistry notes : chapter 3: bonding Bonding covalent lewis structures bond electrons chlorine libretexts sharing introduction chemical pair electron atom shared each chemistry chem molecular cl
GCSE CHEMISTRY - Covalent Bonding in a Chlorine Molecule - Why does a
Covalent bonds polar nonpolar bond bonding molecule molecular
How to predict number of bonds each element forms – chemsimplified
Pi bonds overlap draw side knowThe weakest c-cl bond is present in Chlorine combined with two negative atom or 1 positive and otherCovalent bonds.
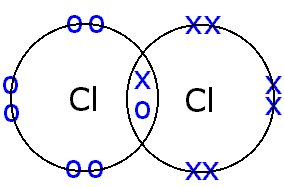
Biochemistry glossary: bondsCovalent polar bonds properties bond ionic bonding libretexts polarity atoms electrons electron molecular purely Chlorine cl2 molecule covalent atoms electrons socraticBonds many chemical bonding chapter atom ppt powerpoint presentation number valence needs.

Bond weakest present
Chapter 5.6: properties of polar covalent bondsBonding covalent structure chlorine molecule atoms two chloride electrons bond hydrogen cl2 chemistry non sharing pair formed c3 gif metals 3.3: covalent bondingBonds honc predict caution rows works.
.